Description
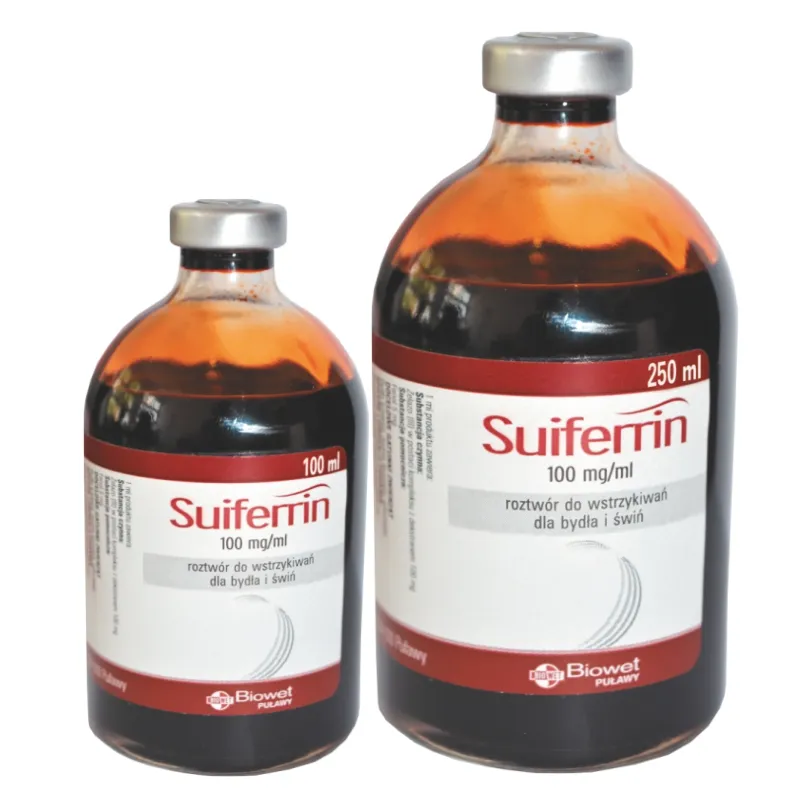
Injection preparation with iron dextran for swine and cattle
Active substance and excipient content
1 ml contains:
Active substance:
Iron (III) in the form of a complex with dextran 100 mg
Excipients:
Phenol 5 mg
Therapeutic indications
Prophylactic and therapeutic use in anaemia resulting from iron deficiency. Suiferrin compensates the shortage of iron in the body, stimulates the haematopoietic system for synthesis of haemoglobin and increases the number of erythrocytes.
Contraindications
Hepatic disorders and renal insufficiency.
Hypersensitivity to iron dextran.
Anaemias not resulting from iron deficiency.
Adverse reactions
In rare cases iron dextran induces symptoms of anaphylactic shock in piglets, and in extreme cases – deaths. The causes might be genetic factors, lack of vitamin E or selenium. Irritation, swelling and brown discoloration of adjacent tissues might occur at the injection site. In the case of serious vitamin E and/or selenium deficiencies in the diet of sows, hypersensitivity to iron manifesting itself with nausea, vomiting and sudden death approximately an hour after administration of products containing iron compounds may occur.
Any adverse reactions emerged after administration of the product or any observed symptoms not listed in the leaflet (including symptoms reported in humans following exposure to the product) should be reported to the competent veterinarian, Marketing Authorization Holder or the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products. The report form should be downloaded from https://www.urpl.gov.pl (Department of Veterinary Medicinal Products).
Amount to be administered per species, method and route of administration
Use intramuscularly or subcutaneously.
Piglets, weaners: 2 ml per animal.
Calves: 4-8 ml per animal.
Indications for proper administration
None
Withdrawal period
Edible tissues: zero days.
Special precautions for storage
Keep out of the reach and sight of children.
Do not store at a temperature over 25°C. Store in the original container in order to protect from light. Do not freeze.
The durability period after the first opening of the immediate container: 28 days
Do not use the veterinary medicinal product after the expiry date stated on the label.
Special warnings
Special precautions for use in animals:
Iron dextran may cause an anaphylactic shock in weaners. The causes might be genetic factors, vitamin E or selenium deficiency. If vitamin E and/or selenium deficiencies are suspected, product containing iron compounds should not be used.
The product must only be administered via recommended routes. Intravenous administration may lead to acute poisoning mainly manifesting itself with an anaphylactic shock. Sudden deaths of animals without prodromal symptoms may occur or symptoms from the nervous system may occur: abnormal balance, progressing depression leading to a coma. After oral administration, bloody vomiting, diarrhoea or constipation may occur.
Special precautions to be taken by the person administering the veterinary medicinal product to animals:
Caution should be taken to avoid accidental self-injection. In the case of accidental self-injection, immediately seek medical advice and show the package leaflet or the label to the physician.
Pregnancy:
Not applicable.
Lactation:
Not applicable.
Interactions with other medicinal products or other types of interactions:
The product should not be combined with oral iron products.
Combining the product with tetracyclines and chelating compounds is not recommended because iron ions may form poorly soluble complexes with them which prevent absorption.
Overdose (symptoms, emergency procedures, antidotes):
In overdose, the following disorders of the gastrointestinal tract might occur: bloody vomiting, diarrhoea or constipation. Iron excess may induce a shock, cardiac disorders leading to collapse, breathlessness and renal insufficiency manifesting itself with oliguria or anuria.
Symptoms of chronic iron poisoning result from hepatic disorders caused by accumulation of iron in hepatocytes and Kupffer cells.
Pharmaceutical incompatibilities:
Since no studies of the conformity of this veterinary medicinal product have been conducted, this product must not be combined with other medicinal veterinary products.
Special precautions for the disposal of unused veterinary medicinal product or waste materials derived from the use of such products
Medicines should not be disposed of via wastewater or household waste.
Ask your veterinary surgeon how to dispose of medicines no longer required. These measures should help to protect the environment.
Available containers:
Glass bottles containing 100 ml of the product packed individually in cardboard boxes.
Shelf life
3 years for a veterinary medicinal product packed for sale. Use within 28 days after the first opening of the immediate container.
For animal treatment only.
Subject to medical prescription – prescription drug
To be administered under veterinary supervision.
Other information
For any information on this veterinary medicinal product, please contact the local representative of the marketing authorisation holder.
Biowet Puławy Sp z o. o.
Arciucha 2, 24-100 Puławy
Tel./fax: (81) 886 33 53, Tel: (81) 888 91 00
e-mail:sekretariat@biowet.pl
2017-03-23 decision
2017-03-23 SPC
CPLW 2017.03.23
27.07.2017 r.