Description
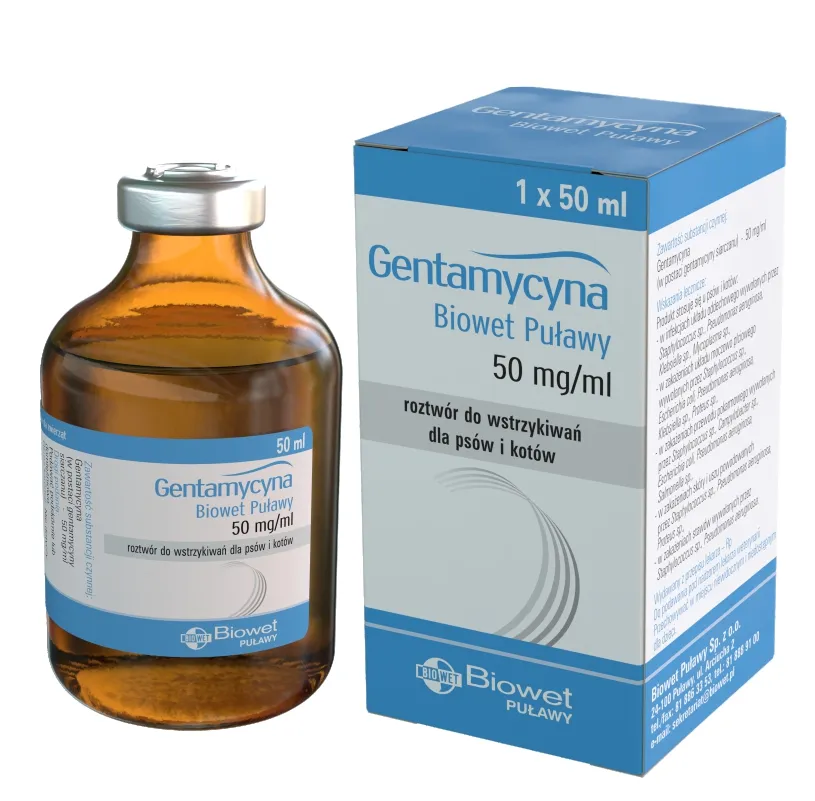
Gentamycyna Biowet Puławy, 50 mg/ml, solution for injection for dogs and cats
Statement of the active substance(s) and other ingredient(s)
Active substance:
Gentamicin (gentamicin sulfate) – 50 mg/ml
Excipients:
Sodium pyrosulfate – 3.5 mg/ml
Disodium edetate – 1.3 mg/ml
Clear. colourless solution.
Target species
Dogs, cats.
Indications
Respiratory infections caused by Staphylococcus sp., Pseudomonas aeruginosa, Klebsiella sp., Mycoplasma sp.,
Genitourinary tract infections caused by Staphylococcus sp., Escherichia coli, Pseudomonas aeruginosa, Klebsiella sp., Proteus sp.,
Gastrointestinal infections caused by Staphylococcus sp., Campylobacter sp., Escherichia coli, Pseudomonas aeruginosa, Salmonella sp.,
Skin and ear infections caused by Staphylococcus sp., Pseudomonas aeruginosa, Proteus sp.,
Joint infections caused by Staphylococcus sp., Pseudomonas aeruginosa.
Contraindications
Do not use in pregnant animals.
Do not use in animals with renal failure.
Do not use in animals allergic to aminoglycosides.
Do not use in severely dehydrated animals.
Special warnings
Special warnings:
None.
Special precautions for safe use in the target species:
Young animals in which renal clearance of gentamicin takes more time than in adult animals are more susceptible to the toxic effect of the product.
In animals less than 2 weeks of age, administer half the recommended doses.
Administration of the product should be based on results of antimicrobial resistance testing on isolates from sick animals. If this is impossible, applied treatment should be based on the local epidemiological information concerning drug susceptibility of the isolated bacteria.
If animal condition requires longer administration of the drug, it is recommended to monitor kidney health by controlling serum urea and serum creatinine concentrations.
Special precautions to be taken by the person administering the veterinary medicinal product to animals:
The product may sensitise the skin leading to development of contact dermatitis. During administration of the product, wear protective clothing and take special precautions. In case of accidental contact with the product, immediately wash away the solution from the surface of skin or mucous membranes. In case of self-injection, hypersensitivity reaction may be elicited. In case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the physician.
Special precautions for the protection of the environment:
Not applicable.
Pregnancy and lactation:
Do not use during pregnancy.
Due to the nephrotoxic effect, use caution in the lactation period, only when the benefit for the mother exceeds the potential risk for the newborn animals.
Interaction with other medicinal products and other forms of interaction:
Gentamicin shows cross-resistance to other aminoglycosides. It acts synergistically with β-lactam antibiotics (especially ampicillin and benzylpenicillin) against enterococci, staphylococci and streptococci. Also, it acts synergistically with vancomycin and rifampicin against streptococci and staphylococci. Cephalosporins and some diuretics enhance nephrotoxicity and ototoxicity of the product. Therefore, it cannot be administered in combination with cephalotin, cephaloridine, etacrynic acid, mannitol and furosemide. Its simultaneous use with vancomycin enhances nephrotoxicity of both drugs. A combination with cisplatin reduces gentamicin elimination, thus posing a risk of nephrotoxicity and hypomagnaesemia. The preparation should not be mixed with solutions of broad-spectrum penicillin, as this may lead to inactivation of aminoglycoside. Its simultaneous use with amphotericin B, cyclosporine, cisplatin, methoxyflurane, acyclovir and non-steroid anti-inflammatory drugs may cause kidney damage. In general anaesthesia, gentamicin administered in combination with cyclopropane may cause apnoea.
Overdose:
Gentamicin toxicity may cause renal failure, neuromuscular block or hearing loss. If any of the above-listed signs occur, discontinue administration of the product.
Major incompatibilities:
Do not use simultaneously with other antibiotics, strong diuretics and drugs likely to cause nephrotoxicity and ototoxicity.
Do not administer in combination with anaesthetics or muscle relaxers.
Adverse events
Long-term administration of the product or gentamicin toxicity may lead to kidney or hearing damage. Intrathecal administration may cause inflammation of nerve roots, fever and chronic pleocytosis.
Reporting adverse events is important. It allows continuous safety monitoring of a veterinary medicinal product. If you notice any side effects, even those not already listed in this package leaflet, or you think that the medicine has not worked, please contact, in the first instance, your veterinarian. You can also report any adverse events to the marketing authorization holder using the contact details found at the end of this leaflet, or via your national reporting system: Department for Documentation Assessment and Pharmacovigilance of Veterinary Medicinal Products, Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, Al. Jerozolimskie 181C, PL-02-222 Warsaw, Tel.: +48 22 49-21-687, Fax: +48 22 49-21-605. https://smz.ezdrowie.gov.pl
Dosage for each target species, routes() and method(s) of administration
Administer the product subcutaneously or intramuscularly in a dose of 0.8 ml/10 kg body weight (which corresponds to 4 mg of gentamicin/kg body weight)
– on the first day of treatment, administer the product every 12 hours,
– consecutive days – once daily every 24 hours.
Administer the antibiotic for 4 to 5 days, in case of urinary infections – for 7 to 10 days.
Urine alkalinization increases antibiotic activity.
Advice on correct administration
None.
Withdrawal period(s)
Not applicable.
Special storage precautions
Keep out of the sight and reach of children.
Store below 25°C. Protect from light. Do not freeze.
Do not use this veterinary medicinal product after the expiry date which is stated on the label.
Shelf life after first opening the immediate package: 28 days.
Special precautions for disposal
Medicines should not be disposed of via wastewater or household waste.
Use take-back schemes for the disposal of any unused veterinary medicinal product or waste materials derived thereof in accordance with local requirements and with any applicable national collection systems. These measures should help to protect the environment.
Ask your veterinary surgeon how to dispose of medicines no longer required.
Classification of veterinary medicinal products
Veterinary medicinal product subject to prescription.
Marketing authorisation numbers and pack sizes
MA number: 281/96
Pack size: 50 ml
Date on which the package leaflet was last revised
14/12/2023
Detailed information on this veterinary medicinal product is available in the Union Product Database (https://medicines.health.europa.eu/veterinary).
Contact details
Marketing authorisation holder and manufacturer responsible for batch release:
Biowet Puławy Sp. z o.o.
Henryka Arciucha 2
24-100 Puławy
Poland
Tel./Fax: + 48 (81) 886 33 53, Tel.: + 48 (81) 888 91 00
e-mail: sekretariat@biowet.pl
Contact details to report suspected adverse events:
Biowet Puławy Sp. z o.o.
Henryka Arciucha 2
24-100 Puławy
Poland
Tel: + 48 (81) 888 91 33, + 48 509 750 444
e-mail: biowet@biowet.pl
SPC 2023-12-14
2025-03-21