Quality certificates
Quality certificates
We invest in quality and safety
In the company Biowet Puławy Sp. z o.o., manufacturing processes are carried out in accordance with the requirements of Good Manufacturing Practice (GMP), resulting from the provisions of Pharmaceutical Law and European Directives. Medicinal products are manufactured under controlled conditions, using appropriate equipment, and the processes are supervised by qualified personnel.
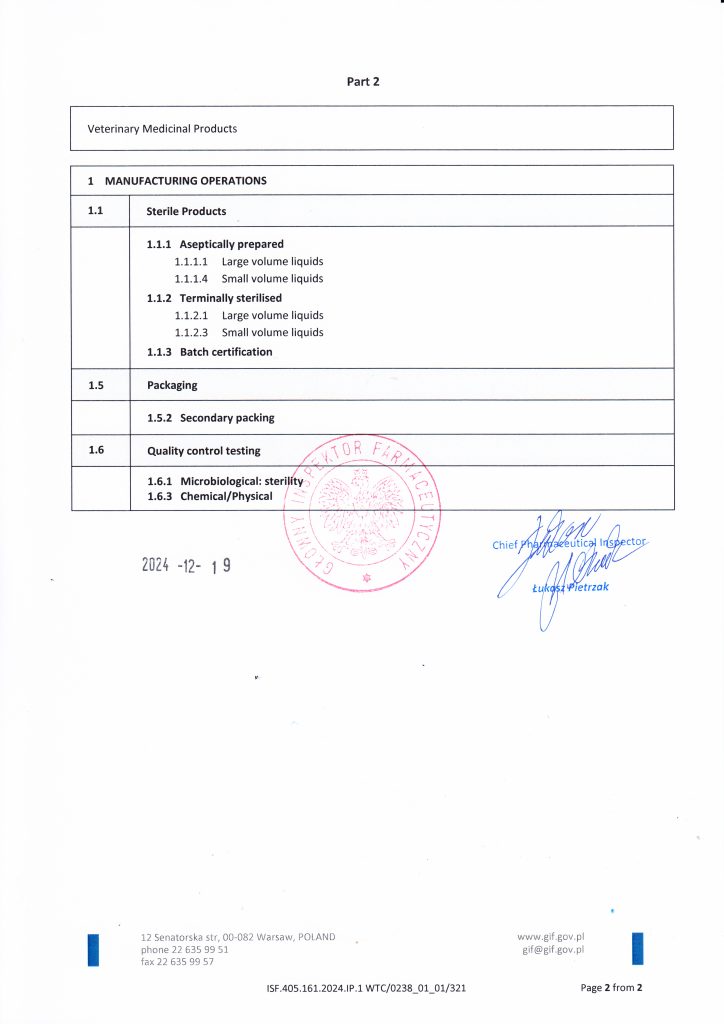
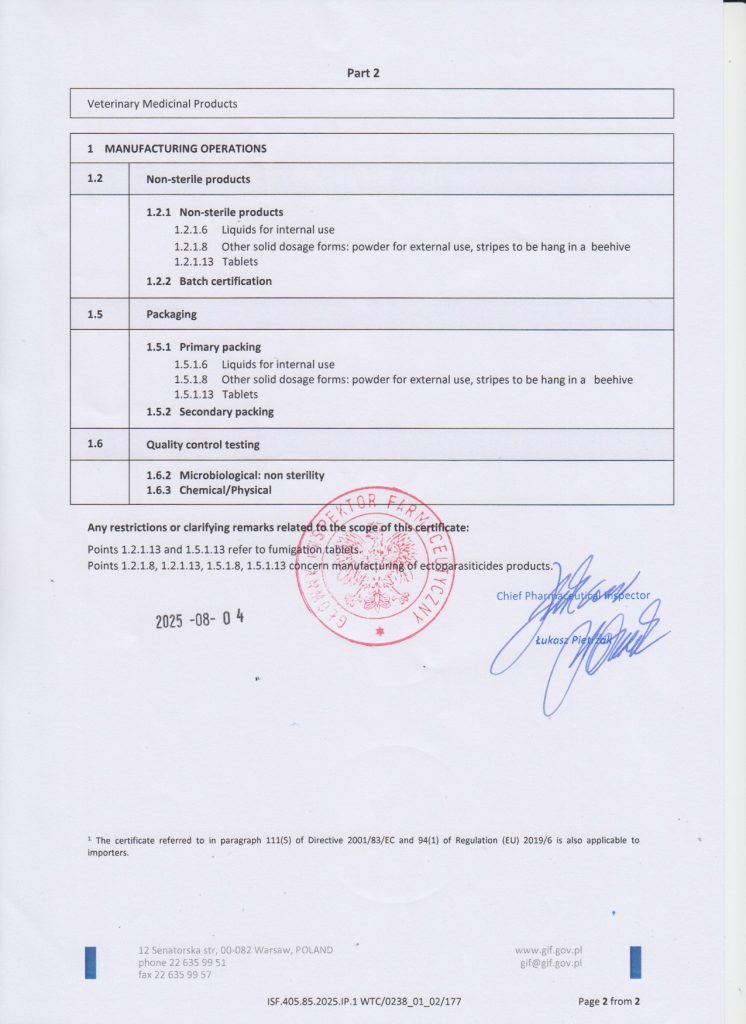
Biowet Puławy holds GMP certificates covering the production of sterile liquid products and non-sterile products, including liquids, powders, hive strips and tablets. The scope of certification also includes biological medicinal products, including immunological products, as well as the conduct of biological testing in the Quality Control Department.
Sterile products
Certificate number
IWSF.405.161.2024.IP.1
WTC/0238_01_01/321
Release date: 2024-12-19 R.
Validity period: 2027-10-11 r.
Non-sterile products
Certificate number
IWSF.405.85.2025.IP.1
WTC/0238_01_02/177
Release date: 2025-08-04
Validity period: 2028-05-09
Biological medicinal products
Certificate number
GIF – IWSF.405.48.2025.IP.1
WTC/0238_01_03/116
Release date: 2025-05-21 r.
Validity period: 2028-02-21 r.
Quality control testing
Certificate number
IWSF.405.48.2025.IP.2
WTC/0238_01_03/117
Release date: 2025-05-21 r.
Validity period: 2028-02-21 r.